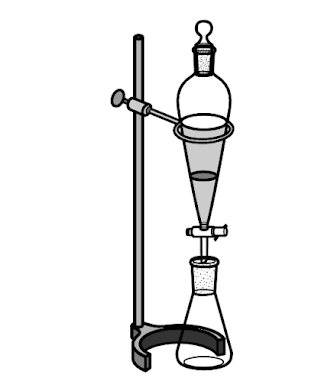
Extraction involves dissolving a compound or compounds either (1) from a solid into a solvent or (2) from a solution into another solvent. Acid-base extraction is the process of separating acids and bases in an organic mixture.
Acid-base extraction is typically used to separate organic compounds from each other based on their acid-base properties.
Generally, most organic compounds are neutral, and therefore more soluble in organic solvents than they are in water. However, if the organic compound becomes ionic, then it becomes more soluble in water. This is useful in extracting an organic acid or base compound from an organic phase to an aqueous phase.
An acid-base extraction can be used to extract carboxylic acids from the organic layer into the aqueous layer. Most organic carboxylic acids are insoluble or slightly soluble in water, but these compounds are highly soluble in dilute aqueous sodium hydroxide because the acid is deprotonated by the base producing the sodium carboxylate salt.
Acid-Base Extraction
The primary goal of food is to promote our health and general well-being. Food science entails comprehending the characteristics, composition, and behaviors of food constituents in different situations, such as storage, handling, and consumption.
October 9, 2022
The Most Popular Posts
-
Ash or mineral content is the portion of the food or any organic material that remains after it is burned at very high temperatures. The a...
-
Crude fiber is a measure of the quantity of indigestible cellulose, pentosans, lignin, and other components of this type in present foods. ...
-
Gelatinization occurs when starch granules are heated in a liquid. It is responsible for the thickening of food systems. The process is an i...
-
Crude fat is the term used to refer to the crude mixture of fat-soluble material present in a sample. Crude fat also known as the ether ext...
-
Vegetables are crucial for maintaining good health due to their rich nutrient profile. Historically, plant-based foods have been integral to...
-
-
-
Most American today are overfed yet undernourished, which eventually leads to obesity and poor health. The answer to those pervasive problem is simply to ...